

2H is the hydrogen nuclide with a neutron and a proton in the nucleus (2H is also called deuterium or heavy hydrogen). Thus the symbol 1H refers to the nuclide of hydrogen with a single proton in the nucleus. Each nuclide is denoted by the element’s chemical symbol (this specifies Z) with the atomic mass number as superscript. The various species of atoms whose nuclei contain particular numbers of protons and neutrons are called nuclides. The total number of nucleons, protons, and neutrons in a nucleus are equal to Z + N = A, where A is called the mass number. The number of neutrons in a nucleus is known as the neutron number and is given the symbol N. Hydrogen (H), for example, consists of one electron and one proton. In the periodic table, the elements are listed to increase the atomic number Z. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor determining its chemical bonding behavior. The configuration of these electrons follows the principles of quantum mechanics. The atom’s chemical properties are determined by the number of protons and the number and arrangement of electrons. Since the number of electrons is responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom. Therefore, the total electrical charge of the nucleus is +Ze, where e (elementary charge) equals 1,602 x 10 -19coulombs. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus.

The total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol Z. The nucleus is composed of protons and neutrons.
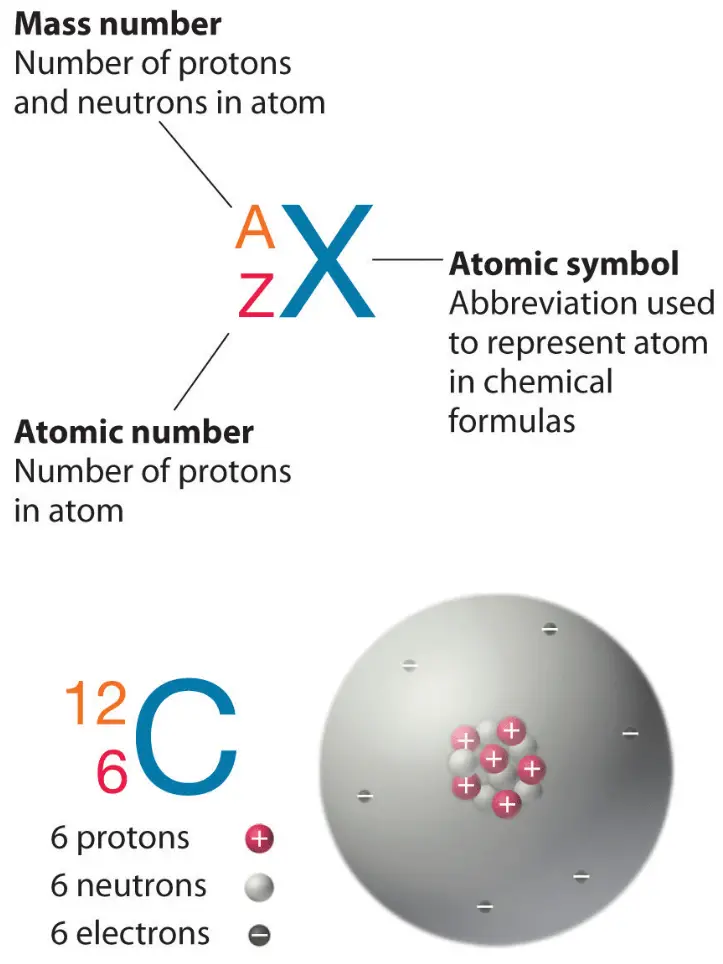
The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The total electrical charge of the nucleus is therefore +Ze. The number of electrons in an electrically-neutral atom is the same as the atomic number. The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol Z.


 0 kommentar(er)
0 kommentar(er)
